|
|
|

|
T-027 / T-027-1ML updated item number format
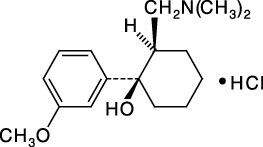
cis-Tramadol HCl
1.0 mg/mL (as free base) in Methanol | Certified Reference Material
|
|
This certified solution standard is suitable for use as starting material in calibrators or controls for a variety of LC/MS or GC/MS applications from forensic analysis and clinical toxicology to urine drug testing and pharmaceutical research. Tramadol is a synthetic analgesic used to treat moderate to moderately severe pain. The drug has a wide range of applications from treatment of rheumatoid arthritis to fibromyalgia.
|
|
|
|
|
| Concentration:
|
1.0 mg/mL (as free base)
|
| Solvent:
|
Methanol
|
| Unit Size:
|
1 mL/ampoule
|
| CAS Number:
|
36282-47-0
|
| Chemical Formula:
|
C16H25NO2 · HCl
|
| Molecular Weight:
|
299.84
|
| Certificate of Analysis:
|
|
| US List Price:
|
|
|
|
|
| D-023 / D-023-1ML |
|
N-Desmethyl-cis-tramadol HCl, 1.0 mg/mL (as free base)
|
|
$42.20
|
|
|
|
|
|
| D-058 / D-058-1ML |
|
O-Desmethyl-cis-tramadol-D6 HCl, 100 μg/mL (as free base)
|
|
$274.00
|
|
|
|
|
|
| D-110 / D-110-1ML |
|
N-Desmethyl-cis-tramadol-D3 HCl, 100 μg/mL (as free base)
|
|
$181.00
|
|
|
|
|
|
| P-071 / P-071-1ML |
|
Pain Management Multi-Component Opiate Mixture-13, 100 μg/mL of each component; 10 μg/mL of Fentanyl
|
|
$449.00
|
|
|
|
|
|
| T-020 / T-020-1ML |
|
cis-Tramadol-13C, D3 HCl, 1.0 mg/mL (as free base)
|
|
$396.00
|
|
|
|
|
|
| T-029 / T-029-1ML |
|
cis-Tramadol-13C,D3 HCl, 100 μg/mL (as free base)
|
|
$72.00
|
|
|
|
|
|
| T-035 / T-035-1ML |
|
O-Desmethyl-cis-tramadol HCl, 1.0 mg/mL (as free base)
|
|
$117.00
|
|
|
|
|
|
| T-119-1ML |
|
Tapentadol-D3 HCl, 1.0 mg/mL (as free base)
|
|
$927.00
|
|
|
|
|
Regulatory Control
▶ USDEA exempt chemical preparation - no USDEA registration or paperwork required
▶ Canada Test Kit Registration # 061-1842 - no Health Canada import authorization required
read more about ordering regulated substances
UN Number, Class, PG
UN2811, class 6.1, PG 3
Safety Data Sheet
HS Code
3822.90.0000
|
▶ A High-throughput Pain Management Panel in Urine Using IONICS 3Q 120 LC-MS/MS and an SLE Sample Prep Platform | IONICS Mass Spectrometry Group inc., 2014
|
|
▶ Direct Analysis of Opioids and Metabolites from Whole Blood Using Ostro Sample Preparation Plates Combined with UPLC/MS/MS for Forensic Toxicology | Jonathan P. Danaceau, Erin E. Chambers, and Kenneth J. Fountain, Waters, 2013
|
|
▶ Direct Analysis of Urinary Opioids and Metabolites by Mixed-Mode µElution SPE Combined with UPLC/MS/MS for Forensic Toxicology | Jonathan P. Danaceau, Erin E. Chambers, and Kenneth J. Fountain, Waters, 2013
|
|
▶ High Throughput Screening and confirmation of 41 Pain Panel Drugs in Oral Fluid by an Integrated On-Line Extraction UHPLC-MS/MS System | Louis Maljers, Zicheng Yang, Bruker Daltonics Inc, 2016
|
|
▶ Proof of Concept for Automated SPE/HPLC/MS/MS Methods to Replace Traditional Immunoassay with MS Confirmation of Driving Under the Influence Samples | Robert M. Sears, Toxicology Technical Leader , Kenneth Lewis, CEO , and Kim Gamble, President, ITSP Solutions, INC., 2014
|
|
▶ Rapid Differentiation of Isobaric Drugs Using a Novel Direct Sample Analysis Source, CID, and Accurate Mass TOF Mass Spectrometry | Robert J. Seward, Bonnie Marmor, and Andrew Tyler, PerkinElmer, Inc., 2014
|
|
▶ Testing and Quantitation of Opioids in Urine and Serum by LC-TOF | Sharanya Reddy, Nonnie Danna, PerkinElmer Inc,, 2014
|
|
|
|
|