|
|
|

|
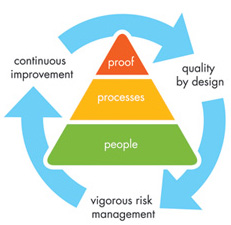
Cerilliant is committed to quality at every level of the organization and sustains
a robust, comprehensive, modern quality system that incorporates quality by design,
risk assessment & management, and vigorous continuous improvement. Our quality foundation
is our people — we have assembled a talented and experienced team with an
unwavering commitment to quality. We build on that through adherence to rigorous
and qualified processes that ensure quality every step of the way and back
it up with fully documented proof, through the use of batch records to provide traceability
of all materials used, equipment utilized and work performed, and our comprehensive
Certificate of Analysis.
Cerilliant’s quality credentials include accreditations to ISO 17034, ISO/IEC 17025 and certification to ISO 9001.
People
People are our foundation. Our deep scientific expertise and diverse backgrounds
bring a wide range of knowledge and best practices to every project. It is this
collective group of experiences and a collaborative culture that allows us to solve
even the most challenging problems.
- Our team’s expertise is leveraged across the organization from product and project
design to execution. We provide comprehensive interdisciplinary solutions to a wide
array of customer needs
- Over 60% of the entire company hold science degrees; more than 25% hold advanced
degrees
- Stringent hiring practices company-wide, including rigorous background checks and
drug screens
- Continuous training and professional development programs ongoing quest for quality
built into our culture – day in and day out we strive to provide the very best products
and services
- Comprehensive and rigorous improvement process that involves every employee and
all facets of organization – we never stop thinking about improving everything we
do and employees are empowered to monitor, maintain and improve at every step
- Cross-functional teams for product design, process improvement and resolution of
problems
- Leadership team that encourages a collaborative, problem-solving culture that fuels
innovation
- Active participation by management in the design, implementation, monitoring and
improvement of the quality system processes
Processes
- Every project is fully and thoroughly documented in a batch record providing traceability
of all components, calibrations and work performed
- All operating procedures, material test specifications, and analytical methods are
fully documented
- Detailed product specifications established prior to production
- Multiple levels of review before, during and after each project
- Quality Assurance team audits each batch record and reviews all data prior to release
- Full qualification of analytical equipment used for release testing of raw materials
and final products
- All vendors and components evaluated for quality prior to acceptance
- Physical quarantine of materials awaiting QC
- Mapped product storage areas with continuous electronic monitoring
- Validated/qualified analytical methods and techniques
- Critical production processes and systems are qualified to ensure quality, accuracy
and repeatability
- Full characterization and qualification of neat materials including identity, chromatographic
purity, residual water, residual solvent, and residual inorganics
- Full certification of solution-based products including purity, concentration, ampoule-to-ampoule
consistency (homogeneity) as well as lot-to-lot consistency against stringent acceptance
criteria
- Real-time assessment of stability and setting of expiry dates and storage conditions
based on scientific data
- Internal audit programs and corrective / preventive action facilitate monitoring
and continuous improvement across all processes and all departments
- Data archival and backup systems and secondary support systems for critical equipment
- Strong safety and environmental management programs
Proof
Cerilliant supports each product produced with a comprehensive
Certificate of Analysis (COA). Cerilliant COAs for fully certified reference
materials include uncertainty information in accordance with ISO 17034 and ISO/IEC
17025 and traceability information for satisfaction of regulatory requirements.
We also provide full details of all analyses including acceptance criteria and actual
results, analytical methods and run conditions, chromatograms and spectral data
for raw materials, and analytical verification of solution purity, concentration
and homogeneity. Custom COAs and reports can be produced to customer specifications
on custom projects. Competency is further demonstrated by our multiple
ISO accreditations which ensure that Cerilliant products and services meet
the highest industry standards accepted across international borders.
|

|
|
|