|
|
|

|
H-003 / H-003-1ML updated item number format
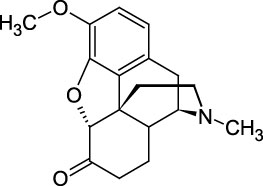
Hydrocodone
1.0 mg/mL in Methanol | Certified Reference Material
|
|
Hydrocodone is a semi-synthetic opioid derived from codeine or thebaine, two naturally-occurring opiates. The drug, also known as dihydrocodeinone, is used as an analgesic to treat pain and as an antitussive for coughing or related conditions. This Certified Spiking Solution® is suitable as a starting material for calibrators or controls in GC/MS or LC/MS testing applications from pharmaceutical research and pain prescription monitoring to clinical toxicology and forensic analysis.
|
|
|
|
|
| Concentration:
|
1.0 mg/mL
|
| Solvent:
|
Methanol
|
| Unit Size:
|
1 mL/ampoule
|
| CAS Number:
|
125-29-1
|
| Chemical Formula:
|
C18H21NO3
|
| Molecular Weight:
|
299.36
|
| Certificate of Analysis:
|
|
| US List Price:
|
|
|
|
Regulatory Control
▶ USDEA exempt chemical preparation - no USDEA registration or paperwork required
▶ Canada Test Kit Registration # 61-1213 - no Health Canada import authorization required
read more about ordering regulated substances
UN Number, Class, PG
1230, 3, II
Safety Data Sheet
HS Code
3822.90.0000
|
▶ A High-throughput Pain Management Panel in Urine Using IONICS 3Q 120 LC-MS/MS and an SLE Sample Prep Platform | IONICS Mass Spectrometry Group inc., 2014
|
|
▶ Advanced Toxicology Testing for Compounds using Core-Shell Technology | Erica A. Guice, Jessica Marsh, Seyed Sajadi, and Matthew Pentis, Western Slope Laboratory, 2012
|
|
▶ An Automated Solid Phase Extraction Method for Opiates in Urine | Jeffery Hackett and Michael Telepchak, UCT, Inc., 2012
|
|
▶ Comparison of Forensic Tandem Mass Spectral Data Obtained on Portable Instrumentation to an Established Reference Library | Adam E. O’Leary, Seth E. Hall, Herbert M. Oberacher, andChristopher C. Mulligan, llinois State University Department of Chemistry, 2014
|
|
▶ Direct Analysis of Opioids and Metabolites from Whole Blood Using Ostro Sample Preparation Plates Combined with UPLC/MS/MS for Forensic Toxicology | Jonathan P. Danaceau, Erin E. Chambers, and Kenneth J. Fountain, Waters, 2013
|
|
▶ Direct Analysis of Urinary Opioids and Metabolites by Mixed-Mode µElution SPE Combined with UPLC/MS/MS for Forensic Toxicology | Jonathan P. Danaceau, Erin E. Chambers, and Kenneth J. Fountain, Waters, 2013
|
|
▶ High Throughput Screening and confirmation of 41 Pain Panel Drugs in Oral Fluid by an Integrated On-Line Extraction UHPLC-MS/MS System | Louis Maljers, Zicheng Yang, Bruker Daltonics Inc, 2016
|
|
▶ LC-MS (TOF) Analysis of Opioids and Opiate-Dependence Management Drugs on Ascentis® Express HILIC | Sigma-Aldrich®, 2014
|
|
▶ LC/MS (TOF) Analysis of Drugs and Their Glucuronide Metabolites on Titan™ C18 after Solid Phase Extraction (SPE) using Supel™-Select SCX, ß-Glucuronidase Enzyme Digestion | Sigma-Aldrich®, 2014
|
|
▶ LC/MS (TOF) Analysis of Opioid Drugs and Glucuronide Metabolites in Urine on Ascentis® Express 2.7 µm HILIC after SPE using Supel™-Select HLB, ß-Glucuronidase Enzyme Digestion | Sigma-Aldrich®, 2014
|
|
▶ LC/MS Analysis of Opioid Glucuronide Metabolites in Urine on Ascentis® Express 2.7 µm F5 after Solid Phase Extraction (SPE) using Supel™-Select HLB | Sigma-Aldrich®, 2014
|
|
▶ LC/MS/MS Analysis of Drugs of Abuse: Generic Screening on Ascentis® Express F5 | Sigma-Aldrich®, 2015
|
|
▶ Proof of Concept for Automated SPE/HPLC/MS/MS Methods to Replace Traditional Immunoassay with MS Confirmation of Driving Under the Influence Samples | Robert M. Sears, Toxicology Technical Leader , Kenneth Lewis, CEO , and Kim Gamble, President, ITSP Solutions, INC., 2014
|
|
▶ Proof of Concept/Comparison of Automated SPE/HPLC/MS/MS Methods to Traditional Immunoassay with MS Confirmation in Death Investigation Cases | Robert M. Sears, Wendy C. Bell , Kenneth C. Lewis, Thurman L. Allsup and Kim Gamble, ITSP Solutions, INC., 2014
|
|
▶ Rapid Differentiation of Isobaric Drugs Using a Novel Direct Sample Analysis Source, CID, and Accurate Mass TOF Mass Spectrometry | Robert J. Seward, Bonnie Marmor, and Andrew Tyler, PerkinElmer, Inc., 2014
|
|
|
|
|