|
|
|

|
M-012 / M-012-1ML updated item number format
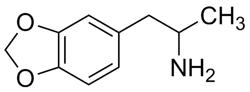
(±)-MDA [(±)-3,4-Methylenedioxyamphetamine]
1.0 mg/mL in Methanol | Certified Reference Material
|
|
A certified solution standard suitable to use in LC/MS or GC/MS applications for clinical toxicology, forensic analysis, or urine drug testing. MDA, or 3,4-methylenedioxyamphetamine, is an illicit recreational drug of the amphetamine and phenethylamine classes. Also known as tenamphetamine, MDA is abused for its psychedelic and hallucinogenic effects, similar to those of MDMA (Ecstasy).
|
|
|
|
|
| Concentration:
|
1.0 mg/mL
|
| Solvent:
|
Methanol
|
| Unit Size:
|
1 mL/ampoule
|
| CAS Number:
|
4764-17-4
|
| Chemical Formula:
|
C10H13NO2
|
| Molecular Weight:
|
179.22
|
| Certificate of Analysis:
|
|
| US List Price:
|
|
|
|
|
| A-050 / A-050-1ML |
|
Amine Mixture-6, 250 μg/mL of each component
|
|
$217.00
|
|
|
|
|
|
| M-010 / M-010-1ML |
|
(±)-MDA-D5 [(±)-3,4-Methylenedioxyamphetamine-D5], 100 μg/mL
|
|
$40.70
|
|
|
|
|
|
| M-011 / M-011-1ML |
|
(±)-MDMA-D5 [(±)-3,4-Methylenedioxymethamphetamine-D5], 100 μg/mL
|
|
$40.70
|
|
|
|
|
|
| M-013 / M-013-1ML |
|
(±)-Methylenedioxymethamphetamine ((±)-MDMA), 1.0 mg/mL
|
|
$34.80
|
|
|
|
|
|
| M-027 / M-027-1ML |
|
(±)-MDA-D5, 1.0 mg/mL
|
|
$156.00
|
|
|
|
|
|
| M-029 / M-029-1ML |
|
(±)-MDMA-D5 [(±)-3,4-Methylenedioxymethamphetamine-D5], 1.0 mg/mL
|
|
$164.00
|
|
|
|
|
|
| M-065 / M-065-1ML |
|
(±)-MDEA [(±)-3,4-Methylenedioxyethylamphetamine], 1.0 mg/mL
|
|
$28.20
|
|
|
|
|
|
| M-067 / M-067-1ML |
|
(±)-MDEA-D5 [(±)-3,4-Methylenedioxyethylamphetamine-D5], 100 μg/mL
|
|
$39.10
|
|
|
|
|
|
| M-068 / M-068-1ML |
|
(±)-MDEA-D5 [(±)-3,4-Methylenedioxyethylamphetamine-D5], 1.0 mg/mL
|
|
$156.00
|
|
|
|
|
|
| M-081 / M-081-1ML |
|
(±)-MDEA-D6 [(±)-3,4-Methylenedioxyethylamphetamine-D6], 100 μg/mL
|
|
$49.90
|
|
|
|
|
|
| M-082 / M-082-1ML |
|
(±)-MDEA-D6 [(±)-3,4-Methylenedioxyethylamphetamine-D6], 1.0 mg/mL
|
|
$406.00
|
|
|
|
|
Regulatory Control
▶ USDEA exempt chemical preparation - no USDEA registration or paperwork required
▶ Canada Test Kit Registration # 61-1280 - no Health Canada import authorization required
read more about ordering regulated substances
UN Number, Class, PG
1230, 3, II
Safety Data Sheet
HS Code
3822.90.0000
|
▶ High Throughput Screening and confirmation of 41 Pain Panel Drugs in Oral Fluid by an Integrated On-Line Extraction UHPLC-MS/MS System | Louis Maljers, Zicheng Yang, Bruker Daltonics Inc, 2016
|
|
▶ Proof of Concept for Automated SPE/HPLC/MS/MS Methods to Replace Traditional Immunoassay with MS Confirmation of Driving Under the Influence Samples | Robert M. Sears, Toxicology Technical Leader , Kenneth Lewis, CEO , and Kim Gamble, President, ITSP Solutions, INC., 2014
|
|
▶ Rapid Enantiomeric Separation of Basic Drugs and Metabolites | John C. Hudson and Hans A. Dewald, Beckman Coulter, Inc., 2010
|
|
▶ Rapid Screening of Amphetamine Drugs in Urine by Positive Ion Electrospray LC/MS/MS | Z. Yang and S. Sadjadi, Agilent Technologies, Inc., 2010
|
|
▶ Testing and Quantification of a Representative Panel of Illicit Drugs in Urine and Serum Using LC-Time-of-Flight Mass Spectrometry | Avinash Dalmia, Noelle M. Elliott, Joanne Mather, Bonnie Marmor, George Perkins;, PerkinElmer, Inc., 2014
|
|
▶ Ultrafast Screen for Amphetamines in Urine Using the Agilent RapidFire High-Throughput Mass Spectrometry System | Kari E. Schlicht and Vaughn P. Miller, Agilent Technologies, Inc., 2013
|
|
|
|
|